Many manufacturers that are new to design controls ask the question, “When should I start design controls?” As with most aspects of the regulation this decision is ultimately up to the manufacturer, but the FDA does give some guidance in the quality systems regulation preamble on this topic:
The Quality Systems Regulation Preamble States:
Comment 62
“The design control requirements are not intended to apply to the development of concepts and feasibility studies. However, once it is decided that a design will be developed, a plan must be established to determine the adequacy of the design requirements and to ensure that the design that will eventually be released to production meets the approved requirements.” (FDA Preamble, 2015)
Comment 65
“Clinical evaluation is an important aspect of the design verification and validation process during the design and development of the device. Because some of the device design occurs during the IDE stage, it is logical that manufacturers who intend to commercially produce the device follow design control procedures. Were a manufacturer to wait until all the IDE studies were complete, it would be too late to take advantage of the design control process, and the manufacturer would not be able to fulfill the requirements of the quality system regulation for that device.” (FDA Preamble, 2015)
So research scientists and engineers can rest assured that design controls are not required during early research, concept or feasibility phases. In recent decades the term “research and development” (R&D) has frequently been lumped together and is often thought of as primarily one phase of product development when in reality they are different. Research typically has very broad discovery targets while only considering some level of efficacy and will often only narrow concepts down to things that work or don’t work without a specific intended use in mind.
Development on the other hand typically begins when the business recognizes a specific market need. From this market need, product scope, plans and requirements can be initiated. The following are indications during development that it is time to begin design controls:
- User needs and design requirements are beginning to be defined
- Management has approved an official project charter to initiate a project to launch the product
- The business begins to form teams which will take the product from feasibility to commercialization
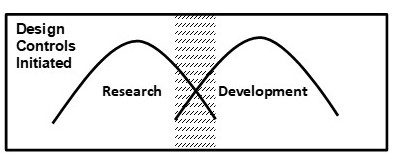
The following graphical models give some clarity as to when design controls typically begin relative to research and development activities.
The two humps in the following figures depict the relationship and the relative amount of research and development activities which occur during the design and development cycle. The shaded area is the typical time when design controls is initiated. Design controls should continue through development until final design validation and design transfer is completed to support a commercialized product which has been approved/cleared by the FDA.