Design controls is a systematic method of developing a product that can potentially maximize the probability of making the “right product” to meet the user’s needs while minimizing the probability of exposing the user to harm.
In layman’s terms, design controls are comprised of the following concepts and activities. This is intended to be a brief and non-inclusive description of design controls activities. To gain a full understanding of design controls, the proceeding chapters should be researched.
- Planning (Design and Development Plan): The process of establishing who, what, how, when and where the device will be developed. Planning should include the identification of design and development activities. It should also specify how different cross functional groups interface during device development.
- Gathering and Defining Requirements (Inputs): Identification of requirements from potential users about what the device should do, how it should perform and in what environment. Translating the user requirements into design specifications is also performed.
- Design the Device: This step involves transforming design requirements into a detailed design. This may include activities such as creating the design virtually on a CAD system (or drawings), defining design schematics, or writing software code.
- Risk Management: During the design process, a risk analysis should be performed to identify risks that may result in harm to the user or patient while using the device. Unacceptable risks may be identified during the risk assessment which should result in a design change to eliminate or at least minimize the risk.
- Design Reviews: These are formal reviews which are typically comprehensive in nature and involve cross functional team review of the design. Design reviews are a forum where teams can challenge the design and/or identify design problems.
- Design Outputs: The documents, drawings, software code etc. that are generated to define the design that are a result of the design process. Outputs also include the results (reports) of design activities such as verification and validation.
- Design Transfer: Design transfer activities ensure production specifications are created which allow for the device to be produced reliably and repeatable within product and process specifications. This is typically achieved by executing process validations, quality checks and other production support procedures which are implemented in the production environment.
- Design Verification: Design verification is typically performed during the development of the device and just prior to final design validation. It is a confirmation that the design meets the design requirements which can be performed by analysis, inspection or testing.
- Design Validation: Performed on the final device configuration to ensure the device performs as required by the user and also to demonstrate that the device is safe and effective.
- Design Change: Process of ensuring that the impact of making a design change is adequately reviewed controlled and approved prior to making the change.
- Design History File (DHF): This is a compilation of documents that were used during development of the device such as plans, design inputs & outputs, protocols, reports, risk assessments, requirements, design changes, etc.
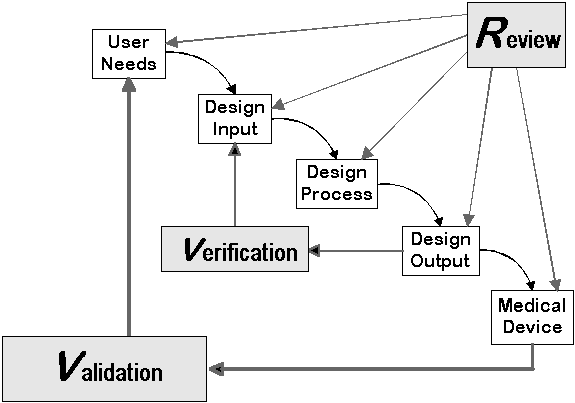
Figure 1 is the traditional waterfall diagram FDA uses to define the relationship between some of the previously listed design control elements. While this diagram can provide a high level understanding of the process, there is significantly more definition and explanation required when actually implementing the process into a medical device manufacturer’s quality system.
(FDA D.C. Guidance, 2015)